
Negative ions are anions.Ĭomplete the following table and consider how you would explain your answers if you were teaching this concept to a peer. When there are more electrons than protons, as in the case of the sulfur ion, the net charge is negative. An electron has a negative charge,so adding one to an atom creates a negatively charged ion or negative ion. This can include ionic compounds such as caesium iodide or. Compounds with iodine in formal oxidation state 1 are called iodides. It is also possible to display the charge symbols in circles.
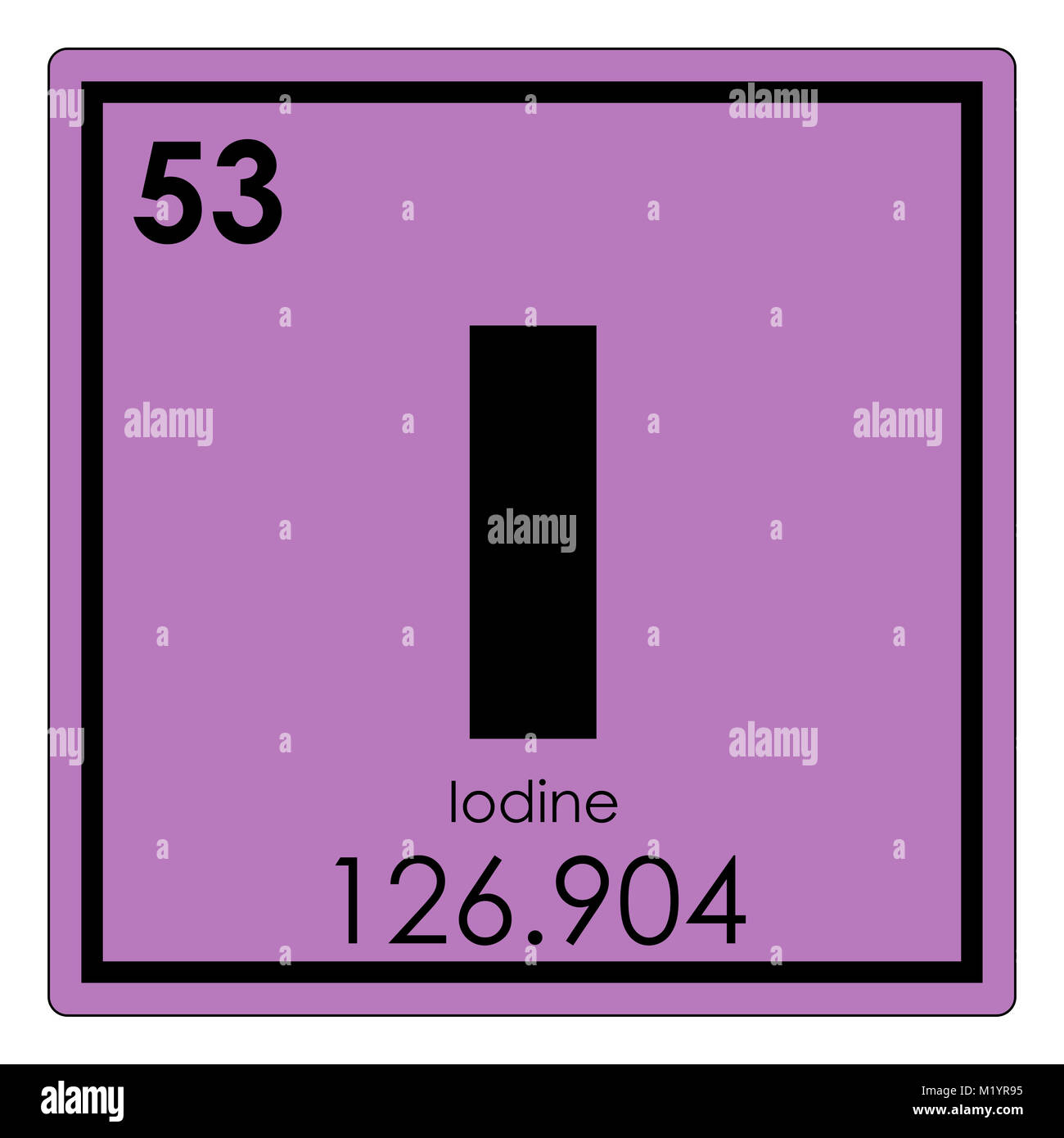
If, however, there are fewer electrons than protons as in the case of the potassium ion, the net charge is positive since there is one more positive proton than negative electrons. An iodide ion is an iodine atom with a 1 charge. lts-iodine lts-krypton lts-lithium lts-mercury Standalone Applications. Since an electron has the same magnitude of charge as a proton but opposite sign (electrons are negative), in order for an atom to be neutral, to have no net charge, the number of protons and electrons are equal to each other. Protons are positively charged particles and the number of protons is determined by the atomic number of the element. What is the element symbol for the halogen in the fifth period on the periodic. Iodide is iodine with a negative charge, and is thus written as I. If the number of electrons changes compared to the number in a neutral atom, the resulting particle is charged. Iodine has the symbol I and atomic number 53. However, the number of electrons in an atom can change. 100 (3 ratings) Iodine has 53 atomic number means it has 53. In a neutral atom, the number of protons and electrons are equal to each other. If the number of protons changes (as you will in see in unit 2 can happen during nuclear reactions) the identity of the atom changes.
It is employed in medicine to monitor thyroid gland functioning, to treat goitre and thyroid cancer, and to locate tumours of the brain and of the liver. An exceptionally useful radioactive isotope is iodine-131, which has a half-life of eight days. \)Īs discussed in the previous section, the number of protons in an atom determines to which element an atom belongs. Other articles where iodine-127 is discussed: iodine: Occurrence and distribution: isotope of iodine is stable iodine-127.


 0 kommentar(er)
0 kommentar(er)
